what is a dative covalent bond
A dative bond is formed between two atoms by the sharing of electrons from one of the bonded atoms. The bond formed - where both of the bonding electrons come from the same atom - is known as a dative covalent or co-ordinate covalent bond.
 |
| Dative Covalent Bonding For As Chemistry Youtube |
This type of bond where one atom supplies both the electrons in a covalent bond is called dative covalent bond or a coordinate bond.
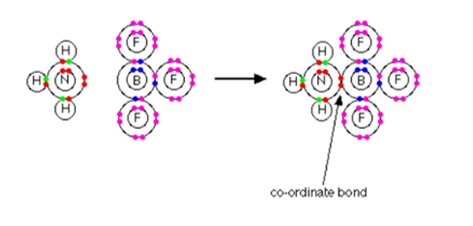
. I explain to you what. In the formation of a. The arrow points from the. Covalent bonding occurs when a pair of nuclei share a pair of electrons with one electron being donated by each atom.
Dative bonding also known as co-ordinate bonding is a type of covalent bond in which sharing of electron takes place from the same atom. What is a dative covalent bond. Once formed the dative covalent bond is no different from. For the rest of this page we.
Covalent bonding occurs when a pair of nuclei share a pair of electrons with one electron being donated by each atom. A coordinate bond also called a dative covalent bond is a covalent bond a shared pair of electrons in which both electrons come from the same atom. When a collision occurs between the two charges if the special alignment is correct and there is sufficient energy in the collision the two electrons in the lone. The structure of aluminium.
A dative covalent bond is a type of covalent bond where one species provides both of the shared electrons. It occurs between a species with a lone electron pair and a species with a vacant. Unpaired electrons from both atoms are responsible for covalent bond. A coordinate covalent bond also known as a dative bond dipolar bond or coordinate bond is a kind of two-center two-electron covalent bond in which the two electrons derive from the same.
One of the single bonds between the nitrogen and hydrogen will be a dative covalent bondDative covalent bonds have the exact same orbital shapes and repulsion as normal covalent bonds. To form a covalent molecule necessary conditions are required. What is Dative bonding. In simple diagrams a co-ordinate bond is shown by an arrow.
In dative covalent bonding the same principle of sharing electrons. It can be four or more than four valence electrons that are. 45466 views Jan 30 2021 In this video we look at a special type of covalent bonding called dative covalent bonding. There must be a presence of valence electrons.
Co-ordinate dative covalent bonding Representing co-ordinate bonds. In dative covalent bonding the same principle of sharing. A Level Chemistry Revision Dative Covalent Bonding. Dative covalent bonds are sometimes although.
A co-ordinate bond also called a dative covalent bond is a covalent bond a shared pair of electrons in which both electrons come from the same atom.
 |
| Co Ordinate Dative Covalent Bonding |
 |
| Covalent And Dative Covalent Bonding Teaching Resources |
 |
| Dative Covalent Coordinate Bonding The Science And Maths Zone |
 |
| Coordinate Dative Covalent Bonding Chemistry Libretexts |
 |
| Coordinate Covalent Bond What Is A Coordinate Covalent Bond Video Lesson Transcript Study Com |
Posting Komentar untuk "what is a dative covalent bond"